Introduction
An increase in gut microbial diversity during Stem cell transplant (HSCT) has been associated with improved treatment-related mortality (TRM) and gastro-intestinal graft versus host disease (GI GVHD). Prebiotics are indigestible carbohydrates rich in fructo-oligosaccharides such as inulin and Galacto- oligosaccharides. Prebiotics are metabolized by the gut bacteria to produce short-chain fatty acids which in turn nourish the gastrointestinal- epithelium. Hence, the implementation of a diet rich in pre-biotics could increase gut microbial diversity and improve outcomes such as GVHD and Overall survival (OS). We report clinical results from single-center Phase I study that investigates the feasibility of consuming a prebiotic rich diet in patients undergoing HSCT.
Methods
Both Allogeneic (Allo) and Autologous (Auto) HSCT Pts >18 yrs were included. Allo Pts undergoing second transplants and needing broad-spectrum antibiotics 1 week before starting the conditioning regimen were excluded. Pts were requested to have at least 2 servings of prebiotic-rich foods such as oats, artichokes an greens daily from the date of conditioning HSCT chemotherapy to Day +100. A list of vegetables and grains with serving size of approximately 5 grams of commercially available fructo-oligosaccharides per day was provided and consumption was recorded with a patient-reported diary. Stool samples were collected before the start of HSCT conditioning regimen, and once a month for 3 months post-transplant. Compliance was defined as consuming 2 servings of prebiotics >/= 80% days of the study period. Secondary endpoints included incidence of acute GVHD, Clostridium difficle infections (C.Diff), Neutrophil (ANC), and Platelet Engraftment. Exploratory endpoints included Meta- genomics sequencing of the stool samples and patient-reported quality of life (QoL) outcomes.
Results
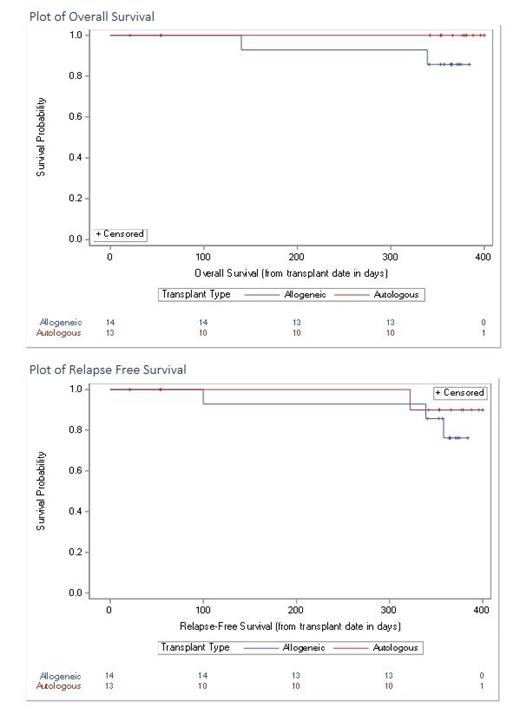
27 Pts, median age 60 yrs were enrolled in this phase 1 study. 5 patients withdrew prior to Day 100 of the intervention. Difficulty in following the diet and maintaining the diary were cited as the main reasons for the withdrawal. 14 were female; 14 pts had Allo HSCT, 5 of whom had Myeloablative conditioning. Only 8 pts were able to comply with the diet (29.6%), 19 were not compliant - of whom 11 (40.8%) pts were not able to comply with the diet and 8 patients were not able to provide a diary. Attempts were made to communicate with them to get an approximation of consumption, none reported > 80% of the study period. None of the patients had C diff infections, 3 of 14 Pts developed acute skin GVHD, grade 1-2; no GI GVHD was reported. Day 100 overall Survival was 100% for both Auto and Allo transplant pts. 1 yr OS 25 pts were evaluable and was 100% and 85.8% in the Auto and Allo Group respectively. 1 pt died of relapsed disease, and another of uncontrolled sepsis at Day +360, after cessation of the diet. Median days for ANC engraftment, and platelet engraftment in the Auto group were 10 days and 16 days; in the Allo group were 17 days and 18 days respectively. The mean percent of change in weight from pre-conditioning to day +100 was -10.26% in the Allo group and -2.5% in the Auto group. FACT-BMT QOL total score decreased by a mean percent of 10.7 % in the Allo pts and improved by 3.8% in the Auto group at Day 100 after transplant from baseline. Meta-genomic analysis of stool samples is currently ongoing.
Conclusion
Results from the Phase I study unfortunately show that a naturally occurring prebiotic-rich diet of green leafy vegetables, pulses, and fiber-rich food is very hard to consume daily in the first 100 days after HSCT. Regional dietary habits, mucositis, and chemotherapy-related change in taste could be contributory factors. However, most importantly implementation of this diet did not result in adverse events such as increased sepsis, GI GVHD, or C Diff infections and reported a remarkable 1 yr OS. Hence further studies to help improve compliance of diet with foods rich in pre-biotics may have a valuable impact on post-Transplant outcomes.
Disclosures
Varadarajan:Kite,: Consultancy, Honoraria, Other: - Advisory board for Follicular Lymphoma .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal